Tuesday, January 25, 2011
Electroplating
Defined as the coating of an object with a thin layer of metal via electrolysis. It is also known as the deposition of a metal coating onto an object by putting a negative charge on it and putting it into a solution which contains a metal salt. The metal salt contains positively charged metal ions which are attracted to the negatively charged object and are reduced to a metal atom.
For instance, the electroplating of an object with copper, using copper(II) sulfate as the electrolyte, copper as the anode and the object to be electroplated as the cathode.
At the anode:
Copper metal is oxidised to form copper ions.
Equation: Cu(s) -> Cu2+(aq) + 2e-
The copper ions enter the solution.
At the cathode:
Copper(II) ions are reduced to form copper metal, which is deposited on the object. There is a net movement of copper from the anode to the cathode. Copper (II) sulphate solution remains unchanged.
Equation: Cu2+ (aq) + 2e- -> Cu (s)
Electroplating is very important and have many uses, for instance to protect the surfaces of other metals, like nickel plating of iron to prevent iron from being oxidised and rusting, to make objects attractive, like electroplating of silver and gold on brass, and to repair machine parts. Sometimes, specific metals like nickel are used to prevent rust, while silver is used for utensils and trophies/metals.
For instance, the electroplating of an object with copper, using copper(II) sulfate as the electrolyte, copper as the anode and the object to be electroplated as the cathode.
At the anode:
Copper metal is oxidised to form copper ions.
Equation: Cu(s) -> Cu2+(aq) + 2e-
The copper ions enter the solution.
At the cathode:
Copper(II) ions are reduced to form copper metal, which is deposited on the object. There is a net movement of copper from the anode to the cathode. Copper (II) sulphate solution remains unchanged.
Equation: Cu2+ (aq) + 2e- -> Cu (s)
Electroplating is very important and have many uses, for instance to protect the surfaces of other metals, like nickel plating of iron to prevent iron from being oxidised and rusting, to make objects attractive, like electroplating of silver and gold on brass, and to repair machine parts. Sometimes, specific metals like nickel are used to prevent rust, while silver is used for utensils and trophies/metals.
Electrolysis of Copper(II) Sulfate using inert electrodes
Expected observations:
Effervescence of colorless odorless gas (oxygen)
Formation of a solid at the cathode, where Copper(II) ion is reduced to form Copper atom.
Intensity of blue solution decreases as Copper(II) has been reduced to Copper atom.
Also, the pH of the solution decreases as the hydroxide ions are discharged while the hydrogen ions remain in solution, along with the sulfate ions.
Selective Discharge and the purification of Copper
Molten sodium chloride only has the presence of two lions, the Sodium and Chloride ions. However, in aqueous solutions, there are ions of the compound (in this case: Sodium and Chloride) and ions of water (Hydrogen and Hydroxide). Therefore in aqueous solutions, there is competition between ions of similar polarity over which would be discharged.
 The rule governing which ion would be discharged is the reactivity series. This is because the more reactive an atom is, the less electronegative it is. As such, it has a lower tendency to gain electrons. In the case of Sodium and Hydrogen ions, since sodium ions are more reactive than hydrogen ions, the hydrogen ions are discharged instead of the sodium ions. Thus we say hydrogen ions are reduced to form hydrogen atoms.
The rule governing which ion would be discharged is the reactivity series. This is because the more reactive an atom is, the less electronegative it is. As such, it has a lower tendency to gain electrons. In the case of Sodium and Hydrogen ions, since sodium ions are more reactive than hydrogen ions, the hydrogen ions are discharged instead of the sodium ions. Thus we say hydrogen ions are reduced to form hydrogen atoms.
Equation: H+ + e- ---> H
Test for Oxygen gas: Insert a glowing splint above solution near the anode end. If splint rekindles, oxygen gas is present.
In dilute solutions, for instance when the concentration is 1.0mol/dm^3, or less, hydroxide ions are discharged except in the cases of copper and silver. This is also known as the concentration effect, another factor affecting the selective discharging of ions during electrolysis. However, concentration only affects the anions and not the cations.
 If we are using inert electrodes, like carbon, Copper(ii) and hydroxide will be discharged to give Copper metal and Oxygen gas. Hydrogen cation and Sulfate will be left behind, so the pH of the solution decreases and becomes more acidic as electrolysis progresses, and the intensity of the blue color fades.
If we are using inert electrodes, like carbon, Copper(ii) and hydroxide will be discharged to give Copper metal and Oxygen gas. Hydrogen cation and Sulfate will be left behind, so the pH of the solution decreases and becomes more acidic as electrolysis progresses, and the intensity of the blue color fades.
Obtaining Copper
Copper is an important element in our daily lives. For instance, it is often manufacturing into wires for copper is a good conductor of electricity and is an abundant element, making it cheap. They are commonly used as domestic plumbing as they do not react with water and are malleable. They can also be used as boilers or heat exchangers as they do not react with water and are good conductors of heat Copper is commonly extracted from copper ores by electrolysis using inert electrodes, at the cathode end, where reduction occurs. Since this copper extracted is not pure enough, it is purified through another process.
Copper Purification process:
The aforementioned setup, an electrolytic cell consisting aqueous copper(II) sulfate with copper electrodes is commonly used to refine copper. Pure copper is used as the cathode, conversely, impure copper is used as the anode.
At the cathode, copper(II) ions are deposited as pure copper.
At the anode, copper goes into solution as Copper(II) ions.
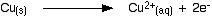
The concentration of the solution remains unchanged as for every copper ion deposited at the cathode to be reduced, another copper ion goes into solution after it is oxidised at the anode. Therefore, there is only a transfer of copper from the anode to the cathode, and the concentration of copper(II) ions in the solution remains the same.
Since the impure copper is purified, there will definitely be impurities. Metals in the impure anode below copper in the reactivity series will not be ionized, remaining as metals.These metals falls to the bottom of the anode as forming "anode sludge" with unreactive materials left from the ore. Metals above copper in the reactivity series will be ionized at the anode by oxidation but will not be discharged at the cathode if their concentration does not become too high. When their concentration becomes too high and the concentration of copper(II) ions fall, the rate of purification decreases as some of the electric current is used to discharge the zinc ions.
 Copper produced through this process is 99% pure.
Copper produced through this process is 99% pure.
 The rule governing which ion would be discharged is the reactivity series. This is because the more reactive an atom is, the less electronegative it is. As such, it has a lower tendency to gain electrons. In the case of Sodium and Hydrogen ions, since sodium ions are more reactive than hydrogen ions, the hydrogen ions are discharged instead of the sodium ions. Thus we say hydrogen ions are reduced to form hydrogen atoms.
The rule governing which ion would be discharged is the reactivity series. This is because the more reactive an atom is, the less electronegative it is. As such, it has a lower tendency to gain electrons. In the case of Sodium and Hydrogen ions, since sodium ions are more reactive than hydrogen ions, the hydrogen ions are discharged instead of the sodium ions. Thus we say hydrogen ions are reduced to form hydrogen atoms. Equation: H+ + e- ---> H
Hydrogen atoms join to form diatomic molecule:
H + H ---> H2
H + H ---> H2
Test for hydrogen gas : Insert a lighted splint above aqueous Sodium Chloride solution near the cathode, lighted splint extinguishes with a "pop" sound.
Both Chloride and hydroxide ions are attracted to the positively charged anode. However, only the chloride ions are discharged, to produce chlorine atoms, Thus, we say chloride ions are oxidised to form chlorine atoms.
Equation: Cl- ---> Cl + e-
Chlorine atoms join to form chlorine gas:
Cl + Cl ---> Cl2
Test for Chlorine gas: Chlorine gas turns damp blue litmus paper red then bleaches it.
Cl + Cl ---> Cl2
Test for Chlorine gas: Chlorine gas turns damp blue litmus paper red then bleaches it.
In the case of anions, halide ions like chloride, bromide and iodide will be discharged. Sulfate ions are not discharged, hydroxide ions are discharged. However, this only applies to concentrated solutions.
4OH- (aq) --> O2(g) + 2H2O (l) + 4e-Test for Oxygen gas: Insert a glowing splint above solution near the anode end. If splint rekindles, oxygen gas is present.
In dilute solutions, for instance when the concentration is 1.0mol/dm^3, or less, hydroxide ions are discharged except in the cases of copper and silver. This is also known as the concentration effect, another factor affecting the selective discharging of ions during electrolysis. However, concentration only affects the anions and not the cations.
The kind of electrodes used also affect the selective discharge of ions. In a usual electrolytic cell, inert electrodes like carbon or graphite would be used. However, in other instances, like the electrolysis of Copper (II) Sulfate solution, Copper is used as the electrode.
In Copper(II) Sulfate solution the ions present are Copper(ii) cation, Sulfate anion, Hydrogen cation and Hydroxide anion.
 If we are using inert electrodes, like carbon, Copper(ii) and hydroxide will be discharged to give Copper metal and Oxygen gas. Hydrogen cation and Sulfate will be left behind, so the pH of the solution decreases and becomes more acidic as electrolysis progresses, and the intensity of the blue color fades.
If we are using inert electrodes, like carbon, Copper(ii) and hydroxide will be discharged to give Copper metal and Oxygen gas. Hydrogen cation and Sulfate will be left behind, so the pH of the solution decreases and becomes more acidic as electrolysis progresses, and the intensity of the blue color fades.If however, copper strips are used as the electrodes, instead of the Copper(II) ions in the solution being discharged, the copper strip at the anode begins eroding and the Copper metal is oxidised to form Copper(II) ions.a
Meanwhile, Copper(II) ions will be travelling to the copper cathode to become Copper metal again. Hence a pink deposit at the anode is observed as newly formed copper metal has a pink appearance. Thus we say copper(II) ions are reduced to form copper metal.
The blue copper sulfate solution will remain blue, since the concentration of Copper(II) ions in the solution doesn’t change. Any increase from the erosion of the anode is balanced by the discharge of ions at the cathode.
Therefore, another factor influencing selective discharge of ions is the type of electrodes used in the electrolytic cell!
---------------------------------------------------------------------------------------------------------------------------------Obtaining Copper
Copper is an important element in our daily lives. For instance, it is often manufacturing into wires for copper is a good conductor of electricity and is an abundant element, making it cheap. They are commonly used as domestic plumbing as they do not react with water and are malleable. They can also be used as boilers or heat exchangers as they do not react with water and are good conductors of heat Copper is commonly extracted from copper ores by electrolysis using inert electrodes, at the cathode end, where reduction occurs. Since this copper extracted is not pure enough, it is purified through another process.
Copper Purification process:
The aforementioned setup, an electrolytic cell consisting aqueous copper(II) sulfate with copper electrodes is commonly used to refine copper. Pure copper is used as the cathode, conversely, impure copper is used as the anode.
At the cathode, copper(II) ions are deposited as pure copper.
At the anode, copper goes into solution as Copper(II) ions.
The concentration of the solution remains unchanged as for every copper ion deposited at the cathode to be reduced, another copper ion goes into solution after it is oxidised at the anode. Therefore, there is only a transfer of copper from the anode to the cathode, and the concentration of copper(II) ions in the solution remains the same.
Since the impure copper is purified, there will definitely be impurities. Metals in the impure anode below copper in the reactivity series will not be ionized, remaining as metals.These metals falls to the bottom of the anode as forming "anode sludge" with unreactive materials left from the ore. Metals above copper in the reactivity series will be ionized at the anode by oxidation but will not be discharged at the cathode if their concentration does not become too high. When their concentration becomes too high and the concentration of copper(II) ions fall, the rate of purification decreases as some of the electric current is used to discharge the zinc ions.
 Copper produced through this process is 99% pure.
Copper produced through this process is 99% pure. Electrolysis of Molten Sodium Chloride
Electrolysis of the ionic compound Sodium Chloride is commonly carried out to obtain Sodium metal. As Sodium is a highly reactive metal, it does not commonly exist as its atom but usually in compounds, like Sodium Chloride, otherwise known as salt. The electric current in this setup are the ions and the electrons in the anode/cathode and the external circuits.
 Since ionic compounds must be in their molton or aqueous forms before they can be electrolyzed, sodium chloride is first heated strongly till it melts. A source of direct current is connected to a pair of inert electrodes (for instance, Carbon) and placed in a solution of molten sodium chloride. The ions are free to move in molten state, and the sodium cations flow towards the cathode while the chlorine anions flow towards the anode. The cathode is negatively charged as electrons are pushed into it by the battery while the anode is positively charged as electrons leave the anode.
Since ionic compounds must be in their molton or aqueous forms before they can be electrolyzed, sodium chloride is first heated strongly till it melts. A source of direct current is connected to a pair of inert electrodes (for instance, Carbon) and placed in a solution of molten sodium chloride. The ions are free to move in molten state, and the sodium cations flow towards the cathode while the chlorine anions flow towards the anode. The cathode is negatively charged as electrons are pushed into it by the battery while the anode is positively charged as electrons leave the anode. At the cathode: The sodium cations are discharged as they take electrons from the negatively charged electrodes to become sodium atoms. Therefore, sodium ions are reduced at the cathode to form sodium atoms.
| Ionic half equation - Reduction at the cathode: | Na+(aq) + e- Na(s) |
At the anode: Chlorine anions that collide with the anode are oxidised to form chlorine atoms.
Ionic half equation - Positive electrode (anode): Cl-(l)
Chlorine atoms combine to form chlorine gas, a diatomic molecule. A color change is observed during the electrolysis of Sodium Chlorine, as a yellow-green gas can be seen at the anode, which is Chlorine gas.
Ionic equation: 2Cl(l)  Cl2 (g)
Cl2 (g)
Combining the two:
Electrolysis of NaCl: | |||
Cathode (-): | Na+ + e- | ||
Anode (+): | 2 Cl- |
Similarly, other molton ionic compounds which are electrolysed will cause the metal to be produced at the cathode and the non-metal at the anode through a redox reaction. Examples of such molten electrolytes include Sodium Iodide (NaI) and Hydrochloric Acid (HCl).
In an Electrolytic cell(voltameter)..
Electrolysis is defined as the decomposition of ionic compounds when electricity is passed through them in molten state or aqueous solutions.
How does this occur? This process is carried out in an electrolytic cell. It consists of two electrodes, metallic rod-shaped conductors which are dipped in a liquid electrolyte and connected by wire to the electron pumper, which in a typical setup is a dry cell or a battery. One of the electrode is an anode, which is connected to the positive terminal, the other, a cathode which is connected to the negative terminal of the battery. This makes the cathode negatively charged (as electrons flow from the negative to the positive terminal, in an opposite direction from conventional current flow), and the anode, positively charged. Since there is presence of ions, there are also cations and anions in an electrolytic cell. Positively-charged CATions are attracted to the negatively charged CAThode while negatively-charged ANions are attracted to the positively charged ANode. There is also a battery, to ensure that there is a difference in charge between the two ends of a conductor. This is to ensure a current can flow . It does so by converting chemical energy into electrical energy.
Redox reactions occur in an electrolytic cell, with reduction occuring at the cathode(electronegative), thus there is a gain of electrons at the cathode. Conversely, oxidation occurs at the anode(electropositive) and there is a loss of electrons at the anode.
(Below: Useful animation showing an electrolytic cell and electrolysis of Copper(II) Sulfate)
 http://www.tutorvista.com/content/chemistry/chemistry-ii/electrolysis/electrolysis-animation.php
http://www.tutorvista.com/content/chemistry/chemistry-ii/electrolysis/electrolysis-animation.php
How does this occur? This process is carried out in an electrolytic cell. It consists of two electrodes, metallic rod-shaped conductors which are dipped in a liquid electrolyte and connected by wire to the electron pumper, which in a typical setup is a dry cell or a battery. One of the electrode is an anode, which is connected to the positive terminal, the other, a cathode which is connected to the negative terminal of the battery. This makes the cathode negatively charged (as electrons flow from the negative to the positive terminal, in an opposite direction from conventional current flow), and the anode, positively charged. Since there is presence of ions, there are also cations and anions in an electrolytic cell. Positively-charged CATions are attracted to the negatively charged CAThode while negatively-charged ANions are attracted to the positively charged ANode. There is also a battery, to ensure that there is a difference in charge between the two ends of a conductor. This is to ensure a current can flow . It does so by converting chemical energy into electrical energy.
Redox reactions occur in an electrolytic cell, with reduction occuring at the cathode(electronegative), thus there is a gain of electrons at the cathode. Conversely, oxidation occurs at the anode(electropositive) and there is a loss of electrons at the anode.
(Below: Useful animation showing an electrolytic cell and electrolysis of Copper(II) Sulfate)
 http://www.tutorvista.com/content/chemistry/chemistry-ii/electrolysis/electrolysis-animation.php
http://www.tutorvista.com/content/chemistry/chemistry-ii/electrolysis/electrolysis-animation.php
Electrolytes versus Non-electrolytes
 Why are we always advised to consume sports drinks after an intense workout? This is because sports drinks contain a high concentration of electrolytes which are essential for the healthy functioning of our body. During an intense exercise, electrolytes like Na+ or K+ are rapidly lost from the body via sweat. Therefore, consuming sports drinks are ideal because they contain many electrolytes necessary for the healthy functioning of cells and tissues, including but not limited to Sodium, Potassium, Chloride, Calcium, Sulfates.
Why are we always advised to consume sports drinks after an intense workout? This is because sports drinks contain a high concentration of electrolytes which are essential for the healthy functioning of our body. During an intense exercise, electrolytes like Na+ or K+ are rapidly lost from the body via sweat. Therefore, consuming sports drinks are ideal because they contain many electrolytes necessary for the healthy functioning of cells and tissues, including but not limited to Sodium, Potassium, Chloride, Calcium, Sulfates. Therefore, electrolytes refer to ionic compounds/ions thats can conduct electricity in the molten state or an aqueous solution, and are themselves decomposed in the process.
(Above: Fig 4.4 showing how strong electrolytes in an electrolytic set-up conducts an electric current which lights up the bulb).
Ionic Compounds in the solid state cannot be considered an electrolyte as they cannot conduct electricity. This is because they exist as a giant ionic lattice of oppositely charged ions held together by strong electrostatic forces of attraction. For instance, in the ionic compound Magnesium Oxide, the Mg2+ and O2- ions are held together closely due this strong electrostatic force of attraction.
In the molten state, the ionic compound has been liquefied by the intense heat applied to the object. As heating the object increases its kinetic energy, the ions vibrate and the rigid ionic structure breaks down to a less-ordered state and is liquefied. As such, the ions can now move freely with electricity and conduct an electric current. Ionic compounds in aqueous solutions can be considered electrolytes. When ionic compounds are in water, they dissociate into their ions. Since there is presence of free moving ions, an electric current can be conducted. Electrolytes can be further classified into strong or weak, which is determined by how much of the ionic compound dissociates into its ions. Therefore, we cannot claim that substances with a greater mole are better electrolytes because not all of the ionic compound might dissociate into its ions.
Non-electrolytes, on the other hand are unable to ionize when in molten or aqueous state. As there are no mobile ions or delocalised electrons in solution, non-electrolytes cannot produce an electric current. Examples of non-electrolytes include organic compounds with hydroxylic groups like alcohol, carbon disulphide, and glucose solution.
Other than the fact that one can conduct electricity and the other cant, electrolytes contain free moving ions in molten or aqueous solution while non-electrolytes do not.
Electrolysis - Specific Lesson Objectives
1. Describe and differentiate between electrolytes and non-electrolytes
2. describe electrolysis as the conduction of electricity by an ionic compound (an electrolyte), when molten or dissolved in water, leading to the decomposition of the electrolyte
3. describe electrolysis as evidence for the existence of ions which are held in a lattice when solid but which are free to move when molten or in solution
4. describe, in terms of the mobility of ions present and the electrode products, the electrolysis of molten sodium chloride, using inert electrodes
5. predict the likely products of the electrolysis of a molten binary compound
6. apply the idea of selective discharge based on
- cations: linked to the reactivity series
- anions: halides, hydroxides and sulfates (e.g. aqueous copper(II) sulfate and dilute sodium chloride solution (as essentially the electrolysis of water)
- concentration effects (as in the electrolysis of concentrated and dilute aqueous sodium chloride)
- (In all cases above, inert electrodes are used)
7. predict the likely products of the electrolysis of an aqueous electrolyte, given relevant information
8. construct ionic equations for the reactions occurring at the electrodes during the electrolysis, given relevant information
9. describe the electrolysis of aqueous copper(II) sulfate with copper electrodes as a means of purifying copper (no technical details are required)
10. describe the electroplating of metals, e.g. copper plating, and state one use of electroplating
Taken from Mrs Chua's SIO and edited
Subscribe to:
Posts (Atom)




